PhD Research
Materials science research at the University of Cambridge with Dr William Jones and was sponsored by the EPSRC and Cambridge Crystallographic Data Centre.
Research focused on common organic molecular fragments with a chloro or methyl group at the same location and chiral configuration (chloro-methyl interchange) and whether they adopt the same crystal structure. Research was in two main parts; (i) How often does isostructural chloro-methyl interchange occur and (ii) is it possible to cause non-isostructural examples to adopt the same crystal structure.
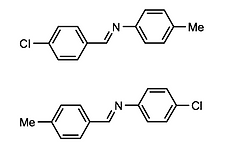

Figure 1. Examples of isostructural chloro-methyl interchanged molecular fragments.
Successful methods used to force non-isostructural examples to adopt the same crystal structure was through co-crystallisation either with; (i) specific ratios of the chlorinated and methylated fragments or; (ii) large molecular hosts capable of minimising the intermolecular effects of the interchanged chloro and methyl groups.
Analytical skills learned:
1. Data mining of the Cambridge Structural Database using an algorithm written in C.
2. Powder X-Ray diffraction for; (i) quantitative analysis of strain in a mixed crystal by observing shifts in miller plane reflections and; (ii) characterisation of polymorphs.
MPhil Research
Research sponsored by Keele University into a new method for the extraction and purification of Azadirachtin using molecular imprinted polymers (MIP).
Azadirachtin is of interest to chemists and biologists due to its insecticide, fungicidal, antibacterial and other bio-activities. Azadirachtin was isolated from the seeds of the Neem tree (A. indica) by David Morgan in 1968 and its molecular structure was discovered about 17 years later. Its isolation from Neem seeds is a costly and laborious process requiring a lot of solvents to separate the Neem oil and polar natural products from the compounds of interest. This means that a more cost effective method of extraction is necessary which prompted the research into the possible use of MIPs for the extraction process.

Figure 2. Azadirachtin molecule
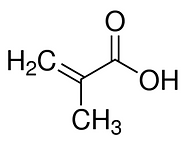
The first trial at creating a MIP for Azadirachtin was done using methacrylic acid as monomer and ethylene glycol dimethacrylate as cross-linker, polymerised in the presence of Azadirachtin. Any left over monomers and cross-linkers were washed from the polymer before testing its affinity for Azadirachtin.
(i)
(ii)

Figure 3. (i) Methacrylic acid; (ii) Ethylene glycol dimethacrylate
Analytical skills learned:
1. HPLC and SFC (super-critical fluid chromatography) which was used to both characterise and purify samples.
2. Hands on nuclear magnetic resonance using a Brucker 800MHz spectrometer.
3. Hands on experience using mass spectroscopy to identify molecular fragments.
4. Infrared spectroscopy to analyse bond vibrations and to characterise molecular fragments.